CONCLUSIONS
Imetelstat, a first-in-class telomerase inhibitor, showed higher rates of red blood cell (RBC) transfusion independence (TI) for ≥8 weeks, ≥24 weeks, and ≥1 year (39.8%, 28.0%, and 17.8%) than placebo (15.0%, 3.3%, and 1.7%) in the IMerge (NCT02598661) phase 3 study of patients with RBC transfusion-dependent non-del(5q) lower-risk myelodysplastic syndromes (LR-MDS) relapsed/refractory to erythropoiesis-stimulating agents (Platzbecker et al. EHA 2023. Abstr S165).
To evaluate the impact of MDS-associated mutations on clinical efficacy of imetelstat, we performed next-generation sequencing of a panel of 36 genes, recurrently mutated in MDS, using DNA samples from peripheral blood collected at study entry. Further analysis of TI responses to imetelstat was performed across different mutation subgroups, defined based on genes involved in different biological functions, including the splicing process, epigenetic modifiers, transcription regulation, and receptors/kinases.
Baseline mutation data were available in 165 of 178 patients (imetelstat, n = 110; placebo, n = 55; 93.2% and 91.7% of total in each group, respectively). Of patients with mutation data, 161 (97.6%) had ≥1 mutation detected, among whom, 75 (70.1%), 33 (30.8%), and 9 (8.4%) patients in the imetelstat group and 38 (70.4%), 15 (27.8%), and 7 (13%) patients in the placebo group had >1, >2, and >3 mutations, respectively. The ≥8-week TI rates in the imetelstat vs placebo groups were 42.7% vs 15.8% ( P = .006) for patients with >1 mutation, 45.5% vs 6.7% ( P = .012) for patients with >2 mutations, and 55.6% vs 14.3% ( P = .089) for patients with >3 mutations, respectively. The ≥24-week TI rates were 26.7% vs 2.6% ( P = .003), 33.3% vs 0% ( P = .014), and 33.3% vs 0% ( P = .117), respectively.
In patients with mutations associated with poor prognosis ( TP53, ETV6, RUNX1, ASXL1 or EZH2), 31.8% and 9.1% of patients in the imetelstat group achieved ≥8-week and ≥24-week TI vs 0 of those in the placebo group. TP53 mutations were detected in 2 patients in each group; both patients in the imetelstat group and none in the placebo group had ≥8-week TI. Among patients with ASXL1 mutations, 5 of 18 patients (27.8%) in the imetelstat and 0 of 6 (0) in placebo group had ≥8-week TI. Among patients with ETV6 mutations, 1 of 2 (50%) in the imetelstat group and 0 of 1 patients in the placebo group had ≥8-week TI. Two patients in each group had RUNX1 mutations; none achieved TI.
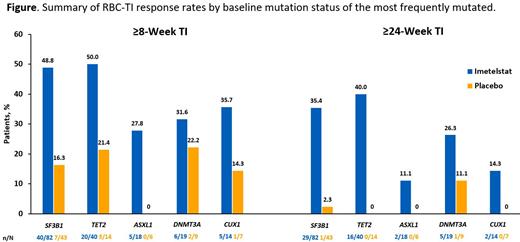
Imetelstat ≥8-week TI rates were 43.8%, 37.7%, 40.0%, and 80.0% for patients harboring mutated genes in the RNA spliceosome, epigenetic modifiers, transcription regulation, and receptors/kinases, respectively; ≥24-week TI was 30.2%, 27.5%, 20.0%, and 80.0%, respectively. The most frequently mutated gene was SF3B1 (125 of 165; 75.8%), the ≥8-week and ≥24-week TI rates were 48.8% vs 16.3% ( P = .001) and 35.4% vs 2.3% ( P < .001) with imetelstat vs placebo. In the imetelstat group, ≥8-week TI was achieved in patients with different spectrum of SF3B1 hot-spot mutations: 2 of 8 patients (25.0%) with E622D, 4 of 7 (57.1%) with R625C/L/G, 7 of 12 (58.3%) with H662Q/N/D/Y, 2 of 2 (100%) with T663P, 2 of 6 (33.3%) with K666R/T/Q/N, 18 of 41 (43.9%) with K700E, 2 of 2 (100%) with A744P, and 1 of 2 patients (50.0%) with E783K. Durable ≥24-week TI was also observed in patients with these hot-spot mutations.
Other genes with mutation frequency >10% were TET2 (32.7%), DNMT3A (17.0%), ASXL1 (14.5%), and CUX1 (12.7%). The ≥8-week TI rates in the imetelstat group vs placebo group were 50% vs 21.4% for TET2 mutations; 31.6% vs 22.2% for DNMT3A mutations; 27.8% vs 0 for ASXL1 mutations; 35.7% vs 14.3% for CUX1 mutations (Figure). The ≥24-week TI rates were 40% vs 0, 26.3% vs 11.1%, 11.1% vs 0, and 14.3% vs 0, respectively, for those mutations (Figure).
Higher RBC-TI rates were observed in patients with various baseline mutational profiles treated with imetelstat compared with placebo in IMerge. While the sample size for specific mutations was small, consistent with the observation that patients with LR-MDS have a low number of specific mutations, TI responses in patients receiving imetelstat occurred regardless of the presence of mutations associated with poor prognosis or the number of mutations. Imetelstat showed comparable TI rates across different molecularly defined subgroups, suggesting that clinical benefit of imetelstat in patients with LR-MDS is independent of the underlying molecular pattern.
OffLabel Disclosure:
Santini:AbbVie: Other: Advisory boards; Janssen: Other: travel grant; CTI: Other: Advisory boards; Otsuka: Other: Advisory boards; Novartis: Other: Advisory boards; BMS/Celgene: Other: Advisory boards; Gilead: Other: Advisory boards; Geron: Other: Advisory boards; Syros: Other: Advisory boards; Servier: Other: Advisory boards. Zeidan:Mendus: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Taiho: Consultancy, Honoraria; Otsuka: Consultancy, Honoraria; Notable: Consultancy, Honoraria; Geron: Consultancy, Honoraria; Foran: Consultancy, Research Funding; ALX Oncology: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; BioCryst: Consultancy, Honoraria; Agios: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Schrödinger: Consultancy, Honoraria; Orum: Consultancy, Honoraria; Kura: Consultancy, Honoraria; Tyme: Consultancy, Honoraria; Regeneron: Consultancy, Honoraria; Syndax: Consultancy, Honoraria; Zentalis: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Boehringer-Ingelheim: Consultancy, Honoraria; Shattuck Labs: Research Funding; BeyondSpring: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Lox Oncology: Consultancy, Honoraria; Ionis: Consultancy, Honoraria; Syros: Consultancy, Honoraria; Astex: Research Funding; Amgen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Chiesi: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Genentech: Consultancy, Honoraria. Fenaux:Janssen: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; French MDS Group: Honoraria; Jazz: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding. Madanat:Rigel Pharmaceuticals: Honoraria; MD Education: Honoraria; OncLive: Honoraria; Novartis: Honoraria; Taiho oncology: Honoraria; Stemline therapeutics: Honoraria; Morphosys: Honoraria, Other: travel reimbursement; Sierra Oncology: Honoraria; Blueprint Medicines: Consultancy, Honoraria, Other: travel reimbursement; GERON: Consultancy. Berry:Geron: Current Employment, Current equity holder in publicly-traded company. Feller:Geron: Current Employment, Current equity holder in publicly-traded company. Sun:Geron: Current Employment, Current equity holder in publicly-traded company. Xia:Geron: Current Employment, Current equity holder in publicly-traded company. Wan:Geron: Current Employment, Current equity holder in publicly-traded company. Huang:Geron: Current Employment, Current equity holder in publicly-traded company. Savona:Incyte: Research Funding; Takeda: Research Funding; TG Therapeutics: Research Funding; AbbVie: Consultancy; BMS/Celgene: Consultancy; Forma: Consultancy; Geron: Consultancy; Karyopharm: Consultancy, Current equity holder in publicly-traded company; Novartis: Consultancy; Ryvu: Consultancy, Current equity holder in publicly-traded company; Sierra Oncology: Consultancy; Taiho: Consultancy; Takeda: Consultancy; TG Therapeutics: Consultancy; Astex: Research Funding; ALX Oncology: Research Funding. Platzbecker:Servier: Consultancy, Honoraria, Research Funding; Celgene: Honoraria; Syros: Consultancy, Honoraria, Research Funding; Jazz: Consultancy, Honoraria, Research Funding; MDS Foundation: Membership on an entity's Board of Directors or advisory committees; Fibrogen: Research Funding; BeiGene: Research Funding; Roche: Research Funding; Merck: Research Funding; BMS: Research Funding; Janssen Biotech: Consultancy, Research Funding; Curis: Consultancy, Research Funding; Silence Therapeutics: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Geron: Consultancy, Research Funding; AbbVie: Consultancy; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; medical writing support, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Research Funding.
imetelstat to treat lower-risk myelodysplastic syndromes relaped/refractory to or ineligible for erythropoiesis-stimulating agents

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal